In June, astronomers reported a disappointing discovery. The James Webb Space Telescope has failed to detect a thick atmosphere around the rocky planet TRAPPIST-1 C, an exoplanet in one of the most attractive planetary systems for the search for extraterrestrial life.
This discovery follows similar news about neighboring planet TRAPPIST-1 B, another planet in the TRAPPIST-1 system. The dim red star is home to her seven rocky worlds, some of which are in the habitable zone. At great distances from the star, liquid water could exist on the surface, allowing otherworldly life to thrive.
The question of what it takes to detect life if it exists is not a new question. But thanks to JWST, it’s finally becoming practical. In the coming years, the telescope will be able to glimpse the atmospheres of several promising planets orbiting distant stars. The first hints of life beyond our solar system may be hidden in these atmospheric chemical reactions. This poses a tricky problem. What qualifies as a true chemical signature of life?
“We’re using very little information about the planets to draw potentially very profound conclusions that will change our view of the universe as a whole,” said Joshua Chrysansen-Totton, a planetary scientist at the University of Washington. It’s something to change.”
To detect such biosignatures, scientists must find clever ways to exploit the limited information they can glean from observing exoplanets.
Chemical context
Even the most powerful telescopes, including JWST, rarely “see” exoplanets. As a rule, astronomers know about these distant worlds only by the flickering of stars.
Instead of observing planets directly, astronomers train a telescope on a star and wait for the planet to “pass,” or pass, between the sun and the telescope. When a planet passes by, a small amount of starlight passes through its atmosphere, dimming the star at certain wavelengths depending on the chemicals in the atmosphere. The resulting dips and peaks in a star’s brightness are like chemical barcodes for passing planets.
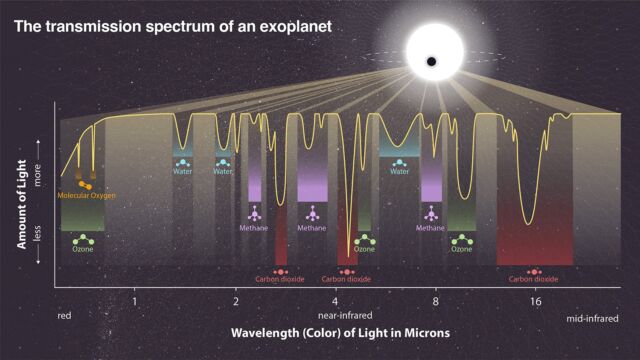
Perhaps the most intuitive method is Look for biosignatures That barcode is apparently meant to look for gases produced by life. For some time, scientists thought that oxygen, which is abundant on Earth through photosynthesis, served as an independent biosignature. However, oxygen can also come from other processes. For example, sunlight can break down water in Earth’s atmosphere.
And this problem is not unique to oxygen. Most of the gases produced by living things can also occur in the absence of life. As such, today’s scientists tend to consider them in context rather than treating single gases as biosignatures in their own right.
For example, methane can be produced with or without life. By itself, it is not a convincing biosignature. But discovering that methane and oxygen exist together “would be very exciting,” says Robin Wordsworth, a planetary scientist at Harvard University. Without vitality, it is very difficult to create that combination. Similarly, work by Chrysansen-Totton and colleagues recently showed that methane can be found with appropriate amounts of other gases, such as carbon dioxide. It would be difficult to explain without life.
Observing how exoplanets’ atmospheres change over time could also provide valuable context that could strengthen weak biosignatures. Seasonal fluctuations in ozone concentration, etc. It may be the fingerprint of my lifeScientists reported in 2018.
Surprise rather than assumption
Of course, “when you’re looking for individual gases like oxygen or methane, there are built-in assumptions about what kind of life there is elsewhere,” says Chrysansen-Totton. . As such, some scientists are developing agnostic biosignatures that do not assume that alien biochemistry is similar to Earth’s biochemistry.
One possibility for an agnostic biosignature is the degree of chemical “surpriseness” of the exoplanet’s atmosphere, or what scientists call chemical disequilibrium.
A near-equilibrium atmosphere is chemically uninteresting, like a sealed gas flask in a laboratory. Of course, nothing on the planet is more boring than a laboratory flask. Chemical reactions in a planet’s atmosphere can be driven by stars, and geological processes such as volcanic activity can increase imbalances and thus increase the chemical wonders of the atmosphere.



