of 2023 Nobel Prize in Chemistry Moungi Bawendi, Louis Brus, and Alexei Ekimov were jointly awarded the award for their discovery and development of quantum dots. These nanoparticles are so small that their size determines their properties. Quantum dots are used in numerous applications, including modern computers, televisions, and LED lighting.
[Related: In photos: Journey to the center of a quantum computer.]
Bawendi Professor at Massachusetts Institute of Technology Blues He is a professor emeritus at Columbia University. Ekimov He works for a company called Nanocrystals Technology in New York state.
“For a long time, no one thought it was actually possible to make particles this small,” says Nobel Committee for Chemistry Chairman Johann Oqvist. said during a press conference. “But this year’s winners were successful.”
Size matters at the nanoscale
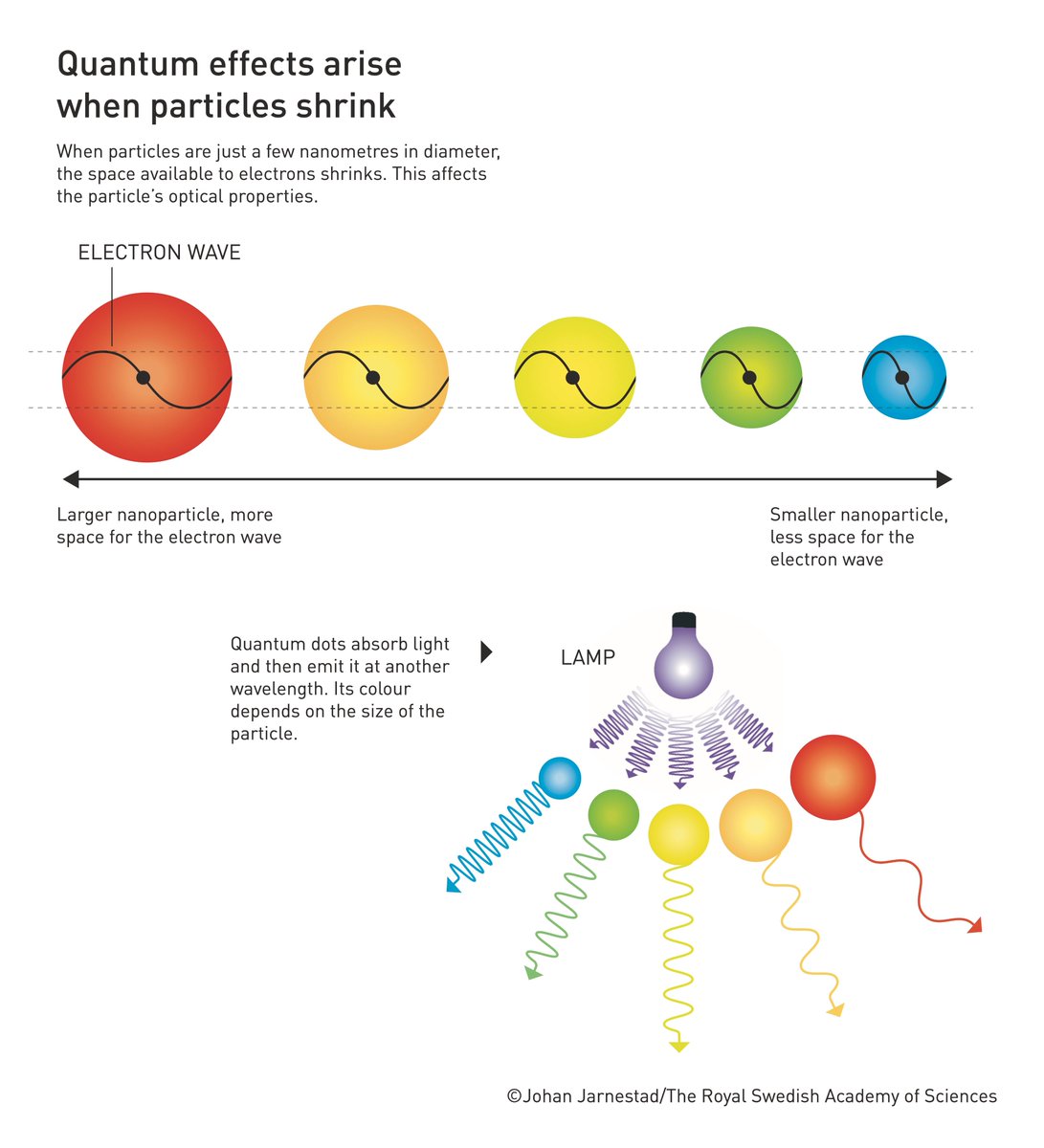
Quantum dots are one of the smallest components of nanotechnology. The properties of an element are usually determined by the number of electrons it has. When that material is reduced to nano-dimensionality, quantum phenomena occur. This means that the properties of an element are governed by the size of the material rather than the number of electrons it has.
Quantum dots are made up of just 1,000 atoms. By comparison, a single quantum dot is to a soccer ball what a soccer ball is to Earth.
The quantum dots created by Bawendi, Brus, and Ekimov are particles small enough that their properties are determined by quantum phenomena. They are one of the smallest but most important particles in nanotechnology.
“Quantum dots have many fascinating and unusual properties. Importantly, they are different [colors] It depends on the size.” Mr. Oqvist said in a statement..
The movement of electrons within quantum dots is highly restricted. This affects the absorption and emission of visible light, allowing very bright colors. The quantum dots themselves are nanoparticles that glow red, blue, or green, and their color depends on the size of the particle. Large dots glow red and small dots glow blue. The color change depends on how electrons behave differently in smaller or smaller spaces.
Great discovery, ultrafine particles
in 1937, physicists theorized that size-dependent quantum effects could occur in nanoparticles. However, few believed it was possible, as it was almost impossible to sculpt in nano-dimensions.
Between early 1980s, Ekimov created a size-dependent quantum effect in colored glass. The color of the glass comes from copper chloride nanoparticles. With this colorful experiment, Ekimov demonstrated that particle size affects the color of glass through quantum effects.
[Related: Quantum computers are starting to become more useful.]
A few years later, Bruce became the first scientist to demonstrate that size-dependent quantum effects in particles are floating freely in a fluid. Brus and Ekimov were actually working independently of each other when they made their initial discovery.
in 1993, Bawendi revolutionized the chemical production of quantum dots. His technique yielded the near-perfect particles needed to use quantum dots in a wide range of applications.
Quantum dots can now be seen on computer monitors and television screens, and can also be useful to biochemists and surgeons Tissue mapping and tumor removal.
Last year’s Chemistry Prize went to a trio of chemists: Carolyn R. Bertozzi, who worked on bioorthogonal chemistry alongside K. Barry Sharpless, and Morten Meldal, who laid the foundations for click chemistry. Awarded.


