digital health company celero system is developing an electronic tablet that can measure heart rate, breathing rate, and core body temperature from inside a person’s stomach. As a first step, the company envisions people with ongoing symptoms using the digital capsule to monitor their vital signs at home. But in the future, they hope to use it as a kind of internal alarm system for drug-related overdoses.
in Small clinical trial published The company tested the device in November on people with sleep apnea. Sleep apnea is a disorder in which breathing stops and starts occasionally during the night. To receive a proper diagnosis, a person often needs to spend a night in the hospital, where electrodes are attached to measure heart rate, breathing, muscle spasms, and brain activity. This is called an overall rating. sleep polygraphy. This is a recipe for a bad night’s sleep, whether you have apnea or not.
Alternatively, patients can choose an at-home test in which they wear a breathing monitor on their finger overnight.But this is still possible cost hundreds of dollars, not always accurate. These wearable devices cannot directly measure breathing, only changes in heart rate that are thought to be caused by breathing. However, the tablets in the stomach do not fall out and the movement of the lungs can be measured internally.
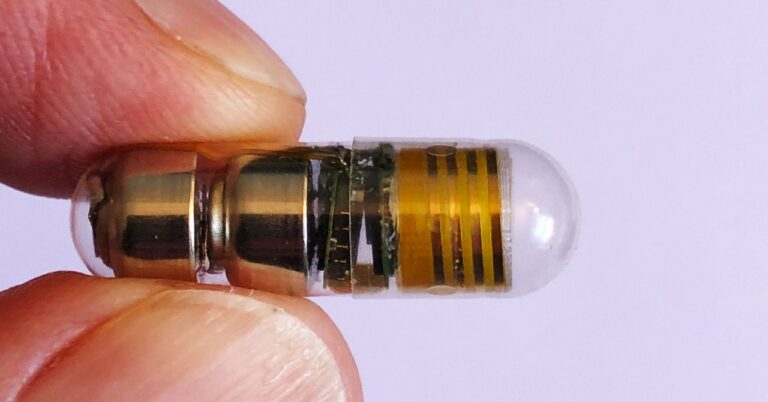
Celero monitoring pills are not actually “pills” in the traditional sense. It’s a biocompatible plastic capsule about the size of a large multivitamin, packed with a tiny sensor, microprocessor, wireless antenna, and battery. Prior to working at Celero Systems, CEO Ben Pless primarily worked in medical implants. First implantable defibrillator. But ingestible devices, or digital pills, have always intrigued her because “they have the potential to be introduced into the body without surgery.” Ingestible products have many of the same benefits as implantable products, such as being discreet and never forgetting to put them on, “except you’re implanting it with a glass of water instead of a surgeon,” he says.
The capsule remains intact during its journey through the digestive system, keeping all electronics safe until it ends up in the toilet a few days later. All the measurements, meanwhile, are transmitted wirelessly to a laptop and can be accessed by researchers, doctors, and even patients. As far as the press knows, Celero’s ingestible device is the first to monitor cardiac and respiratory activity in humans.
In this study, 10 sleep apnea patients at the West Virginia University (WVU Medical Sleep Assessment Center) swallowed a pill before a regularly scheduled sleep study, and researchers found that the pill readings were We made it possible to see how it compares to the gold standard polysomnogram. Accuracy was similar, with an error of only about one breath per minute, which was more than accurate to detect respiratory depression. No one reported any side effects or discomfort, and post-study scans confirmed that all tablets were taken safely within a few days.