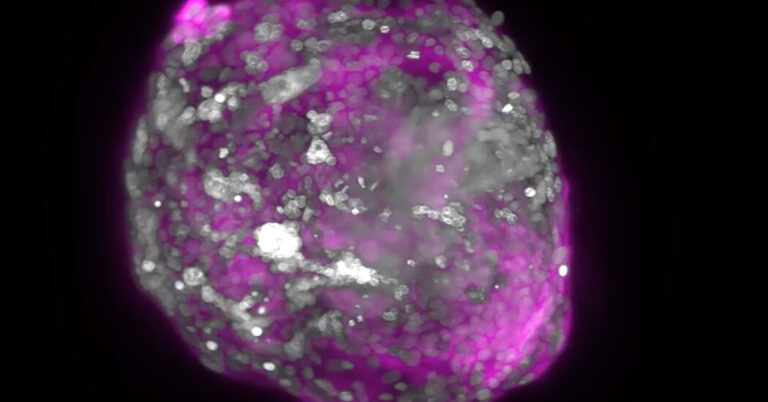
During the first week, a fertilized human egg develops into a hollow sphere of 200 cells and implants in the uterine wall. Over the next three weeks she divides into different tissues of the human body.
And that crucial few weeks remain a black box for the most part.
“We know the basics, but we don’t know the very details at all,” said Jacob Hanna, a developmental biologist at the Weizmann Institute of Science in Israel.
Dr. Hannah and many other biologists are trying to find out more by creating models of human embryos in the lab. They induce stem cells to organize into clumps that bear some of the key characteristics of a real embryo.
this month, Dr. Hannah’s team in Israelas well as a group of UK, America and China, all released reports on these experiments. Although the study has not yet been published in a scientific journal, it has generated intense interest from other scientists, and it is hoped that advances like this may finally solve some of the mysteries of early human development. I’ve been expecting it for many years.
Ethicists have long warned that the advent of embryo models would complicate the already complicated regulation of this research. But the scientists involved in the new study were quick to emphasize that they didn’t create real embryos, and that stem cell clumps could never produce humans.
“Our aim was never for human reproduction,” said Tianqing Li, a developmental biologist at China’s Kunming University of Science and Technology, who led one of the new studies.
Instead, Dr. Lee and his fellow scientists hope that embryonic models will lead to new treatments for diseases such as infertility and even cancer.
“We’re not doing it to create life, we’re doing it to save life,” says Magdalena Zernika-Goetz, a developmental biologist at the University of Cambridge and Caltech, who led another effort.
For decades, the only human embryos that developmental biologists could study were specimens taken from miscarriages or abortions. As a result, scientists were left with deep questions about the beginning of human development. 30% of pregnancies fail in the first week and another 30% during implantation. Researchers are at a loss to explain why most embryos do not survive.
After the development of in vitro fertilization in the 1970s, scientists began studying embryos provided by fertility clinics. Some countries banned research, others allowed it to continue, usually limited to 14 days. By then, the human fetus will begin to acquire some of its key features.a structure called primitive streakFor example, organize the placement of the body from head to feet.
The 14-day rule has long been controversial because no one can keep an embryo alive for more than a few days after fertilization. Things got more complicated in 2016 when Dr. Zernika-Goetz’s group and another team succeeded in keeping the embryos alive. close to the 14th mark. The embryo did not survive long because the scientist destroyed it.
This achievement has led scientists to discussion Embryos may grow beyond 14 days. But even if these experiments were legalized, they would still be difficult to carry out due to the scarcity of donated embryos.
In recent years, researchers have sought easier ways to study embryos by creating models of them in the lab. Scientists have taken advantage of the fact that stem cells can transform into new types of tissue when given the right environmental conditions.
Adults have stem cells in only a small part of their bodies. in the skinFor example, stem cells generate a variety of new cells that heal wounds. In the early embryo, on the other hand, all cells have the potential to transform into different tissues.
last year, Dr. Zernika Goetz’s team and Dr. Hannah’s team An embryonic model was created using mouse embryonic stem cells. Since then, they and other scientists have tried to do the same with human embryonic stem cells.
Each team used a different method, but they all utilize the same underlying biology. By the time a human fetus implants in the uterus, its cells begin to diverge into different types. One cell type continues to generate the cells of the body. Other types produce tissue that surrounds the embryo during development, such as the placenta. These cell types send each other molecular signals that are essential for their development.
The researchers induced stem cells to mimic some of these cell types and mixed them together. Cells swarmed and formed clusters spontaneously. Cells destined for the embryo clustered in the center, while other types of cells migrated to the outside.
As the cells communicated with each other, they divided and formed new structures that resembled parts of the embryo. For example, Dr. Mo Ebrahimkani, a developmental biologist at the University of Pittsburgh, and his colleagues observed yolk sac formation in experiments. From the yolk sac, the development of progenitor cells of blood cells was also observed.
Dr. Zernicka-Goetz and colleagues similarly observed the development of cells resembling egg and sperm precursors.
“This was really thrilling,” Dr. Zernika-Goetz said. “Sometimes it’s hard to believe that these stem cells are growing into these structures.”
If scientists could create accurate and reliable models of embryos, they would be able to perform large-scale experiments to investigate potential causes of pregnancy failure, such as viral infections and genetic mutations.
The model could lead to other medical advances, said Ino Hyun, a member of the Center for Bioethics at Harvard Medical School, who was not involved in the new study.
“Once we have the embryo model properly and can trust it, it could be an interesting way to screen drugs that women take during pregnancy,” she said. “That would be a huge benefit.”
Dr. Hanna and Dr. Ebrahim Khani also noted the potential for using embryonic models as new stem cell therapies for diseases such as cancer.
In a conventional stem cell transplant, doctors remove blood stem cells from the bone marrow before using radiation or chemotherapy to kill the cancer cells. It then puts the healthy cells back into the body.
Unfortunately, this method is not very effective. success rate. Some researchers suggest that early forms of stem cells are likely to cure patients.
Embryonic models may allow doctors to rewind time. Researchers take skin cells from patients and apply chemicals to make them look like stem cells. Other chemicals can turn these stem cells into embryonic models that can grow into the early blood cells that patients need after transplantation.
Alison Muotri, a developmental biologist at the University of California, San Diego, who was not involved in the new study, cautioned that the new study is only preliminary. First, although this technique sometimes yielded embryo-like clusters, it often failed.
“The research is in a very early stage and current methods are by no means reliable,” said Dr. Muotri.