New research reveals genetic variations in the GIP receptor that may help fight obesity by altering metabolism. Mice with this mutant processed sugar more efficiently and remained lean. This finding, along with the observation that the variant affects insulin release, opens new avenues for obesity treatment and highlights the importance of understanding genetic differences in response to weight loss drugs.
Research from Weill Cornell Medicine shows that genetic variations in the GIP receptor may boost metabolism and increase resistance to obesity. insulin It could be liberating and lead to new treatments.
Preclinical studies by Weill Cornell Medicine researchers show that certain human genetic variants in receptors that stimulate insulin release may make individuals more resistant to obesity. The researchers found that this variant behaved differently inside cells, which may contribute to more efficient metabolism.
Insights into obesity resistance
Research published in journals molecular metabolism The Nov. 2 paper provides new insights into how human genetic variation influences an individual’s susceptibility to weight gain. Researchers have developed mice with a human genetic mutation in the glucose-dependent insulinotropic polypeptide (GIP) receptor, which is associated with lean body mass index (BMI). They found that the mice were able to process sugar better and stay leaner than mice with another, more common variant of the receptor. The discovery could point to a new strategy to treat obesity, which affects more than 100 million adults in the United States, according to the Centers for Disease Control and Prevention.
“Our study shows how basic science research can yield important insights into complex biology,” said the study’s lead author, a professor of cardiothoracic surgery and of Weill Cornell Medicine. said Dr. Timothy McGraw, professor of biochemistry. “These GIP receptors and their behavior at the cellular level have profound effects on metabolism and weight regulation.”
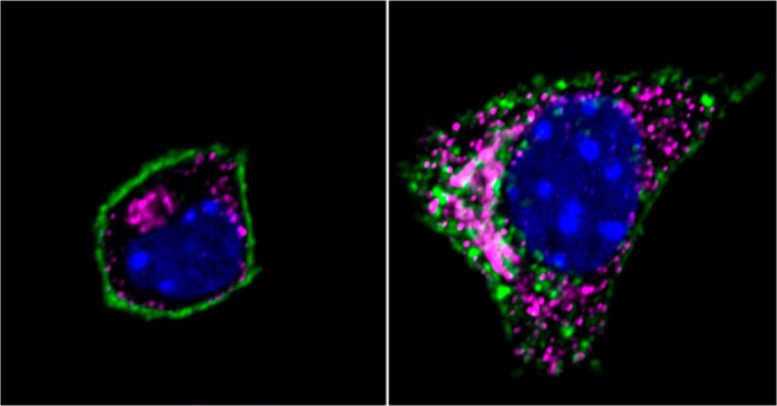
Image of an insulin-producing beta cell shows the GIP receptor Q354 variant (green), Golgi network (magenta), and nucleus (blue). Left: GIP receptors are localized to the plasma membrane surrounding the cell. Right: After the receptor binds to her GIP hormone, it moves inside the cell to the Golgi network. Credit: Weill Cornell Medicine
Genetic variants of the GIP receptor
What is genetic variation? DNA A naturally occurring sequence among individuals within a particular population. Genome-wide association studies, which use statistics to carefully link genetic variation to specific traits, show that about 20 percent of people of European descent have one copy of the GIP receptor with the Q354 gene variant, and about 5 percent have one copy of the GIP receptor with the Q354 gene variant. It has been shown to carry two copies of the mutation. GIP receptors interact with hormones released in response to postprandial glucose levels. “Study suggests that people with at least one copy of this GIP receptor variant have altered metabolism and reduced risk of obesity,” said the lead author of the study. said author Lucy Yamin, Ph.D., a postdoctoral fellow in biochemistry at Weill Cornell Medicine.
To understand how this genetic variation reduces the risk of obesity, the research team used CRISPR-Cas9 technology to introduce mutations into the gene encoding the GIP receptor, similar to its human version. genetically manipulated mice. They found that female mice with this mutation were leaner on a typical mouse diet than their female littermates without it. Male mice with the genetic mutation weighed about the same as their littermates without the genetic mutation while on a normal diet, but due to the genetic mutation, the mice were fed a high-fat diet that caused their littermates to become obese. Weight gain in cases was prevented.
“We discovered that one amino acid changes. acid “The GIP receptor gene had a systemic effect in terms of body weight,” Dr. Yamin said. Mice with this mutation were more sensitive to the GIP hormone, which controls blood sugar levels and causes the release of insulin, which helps the body convert food into energy.
What benefits does the variant offer against obesity?
The researchers compared what happened to mouse cells with and without the mutant when exposed to glucose or the GIP hormone. Pancreatic cells in mice with a genetic mutation produced more insulin in response to both glucose and the GIP hormone. This may explain why pancreatic cells are better at handling glucose.
“What’s interesting about these receptors is that the location of the receptor within the cell has a huge impact on how the signal is transmitted and its activity,” Dr. McGraw said. He explained that when the GIP hormone binds to its receptor, the receptor moves from the cell surface to the inside of the cell. When the GIP hormone is eventually detached from the receptor, the receptor returns to the cell surface.
The research team found that a variant of the GIP receptor remained in a cell compartment four times longer than the typical receptor. Dr. McGraw suggested that this could allow the receptor to send more messages to machinery within the cell, helping it process sugar more efficiently.
Future research and clinical implications
Still, further studies are needed to confirm the effects of this variant on receptor behavior. The researchers also want to know whether there are differences in the behavior of receptors in other types of cells, such as brain cells, which play an important role in regulating hunger.
Recently, the Food and Drug Administration approved several weight loss drugs that mimic the body’s natural hormones and interact with receptors such as GIP, including semaglutide (Wegovy) and tirzepatide (Zepbound). For this reason, there is growing interest in researching new ways to target GIP receptors in obesity.
“Our research suggests that the translocation of receptors from the cell surface to the interior is a key element in the control of metabolism. Therefore, drugs that can modulate the behavior and location of GIP receptors could be used to treat obesity. “This could be an important new tool to combat this,” Dr. Yamin said.
Meanwhile, Dr. McGraw noted that it is essential to understand how people with different genetic variations in the GIP receptor respond to currently available weight loss drugs. “A better understanding of how different variants of the receptor affect metabolism could enable precision medicine approaches to weight loss, where specific drugs are matched to genetic variants. “Maybe,” he said.
Reference: “Spatiotemporal control of GIPR signaling impacts glucose homeostasis, as revealed by studies of common GIPR mutants” Lucie Yammine, Belén Picatoste, Nazish Abdullah, Rosemary A. Leahey, …Timothy E. McGraw, November 2023 molecular metabolism.
This work was supported by National Institutes of HealthR01 DK096925 and R01 DK125699.



