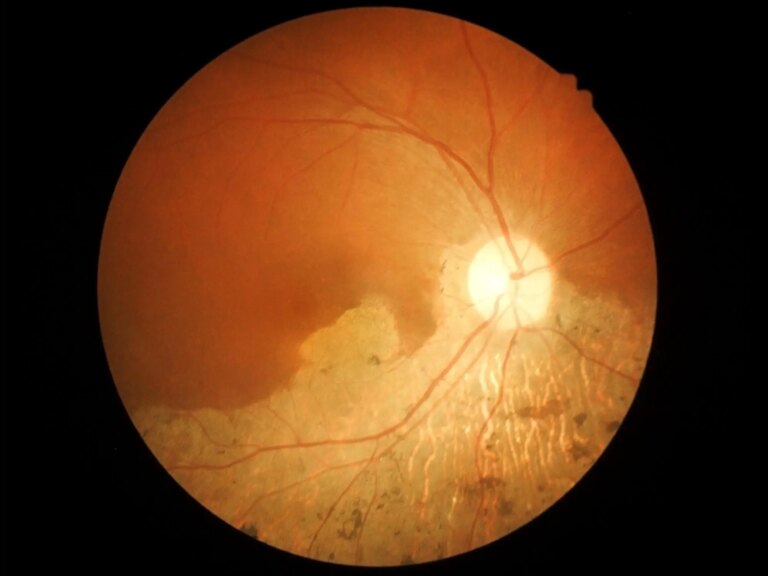
A National Eye Institute study identified a rare genetic mutation that may reveal the mechanism behind age-related macular degeneration (AMD), a common cause of blindness in the elderly. These variants create malformed proteins that disrupt the stability of the Membrane Attack Complex (MAC) and can cause a chronic inflammatory response in the retina. The findings, published in iScience, suggest that MAC is a potential therapeutic target for AMD.
A very rare genetic mutation indicates a potential cause of age-related macular degeneration.
A study from the National Eye Institute (NEI) identified a rare genetic mutation that may point to one of the common mechanisms causing age-related macular degeneration (AMD), a common cause of vision loss in the elderly. identified.
This variant produces a malformed protein that alters the stability of the membrane attack complex (MAC) and can lead to a chronic inflammatory response in the retina.Findings published in the journal eye sciencepoints to MAC as a potential therapeutic target for delaying or preventing the onset of AMD.The NEI is part of the National Institutes of Health.
There are many known genetic mutations that increase or decrease an individual’s risk of developing AMD. However, each of these genetic alterations has a small effect on AMD.
Anand Swaroop, Ph.D., director of the NEI’s Neurobiology, Neurodegeneration and Repair Laboratory and the lead author of the study, led Michael Klein to discover genetic variants and proteins directly related to the disease. I embarked on a joint work with a medical doctor. He is an AMD clinician majoring at the Oregon Health and Science University (OHSU) in Portland. Klein has collected clinical information on hundreds of patients, not just families with many AMD patients. Swaroop, Klein and colleagues looked for families with very rare AMD-causing variants, in which the genetic variant’s influence is so strong that the variant directly affects protein structure and function. Rare variants of this type can reveal the underlying cause of the disease.

The complement membrane attack complex is a pore that inserts into the cell membrane. This complex consists of up to 18 C9 subunits (purple), C5, C6, and C7 subunits (various shades of green), and C8-alpha, C8-beta, and C8-gamma subunits (red). shades). The side (left) and top (middle) views show the split washer configuration of the complex. The C8 subunit connects the C9 ring with the rest of the complex. Very rare mutations in C8-α at arginine 444 and C8-β at residue aspartate 382 (right, highlighted in yellow) appear to stabilize or destabilize the membrane attack complex. Altered stability of the membrane attack complex can lead to chronic inflammation of the retina. Credit: National Eye Institute, NIH
“Although many genetic variants are known to affect AMD risk, only a few directly point to protein changes that may cause AMD,” Swaroop said. “By examining large families with ultra-rare variants that closely track the disease across generations, we are able to identify two proteins that may be the driving force behind AMD pathology in affected patients.” We found that these proteins could be targets for future medicines.”There are currently several treatments to slow vision loss, but AMD wet foamthere is no cure for most patients and no cure for the disease.
Swaroop, Klein and colleagues found that in four families, AMD patients harbored mutations in one of two proteins that form part of the MAC, C8-alpha and C8-beta. The team found that all four AMD family variants affected the ability of the C8 proteins to stick together. This can change the behavior of the MAC on the retina of the eye.
The MAC forms a circular pore closed at one end by the C8 protein. MAC pores allow the flow of ions through the cell’s outer membrane. This pore is the final step in the “complement cascade”, part of the immune system that helps the body defend against pathogens. Scientists initially thought that the sole function of MACs was to insert into bacterial cell membranes and kill pathogens, but recent evidence suggests that MACs are complex in regulating inflammatory processes in tissues such as the retina. shows that it is doing its job.
NEI genetic data Age-related eye disease research suggested a role for the C8 protein as well as other proteins upstream in the complement cascade in AMD. Since the MAC is the final step in the complement cascade, variants affecting either complement protein may leak to alter MAC function. Researchers believe that too much or too little stable MAC in the retina can cause destructive inflammation, which accelerates the progression of AMD.
“Given that the MAC is the end point of the immune system’s complement pathway and the very strong association between these rare variants and disease, targeting the MAC is a promising candidate for controlling AMD.” We think it could be a more effective strategy,” Swaroop said. “Small molecule drugs may control the pro-inflammatory intensity of the MAC and, from there, slow the progression of AMD.”
See also: “An ultra-rare complement factor 8 coding variant in a family with age-related macular degeneration” Lina Zellinger, Tammy M. Martin, Jayshree Advani, Laura Campello, Milton A. English, Alan Kwon, Claire Weber, Jennifer Miko Ski, Yuri V. Sergeev, Robert Fariss, Emily Y. Chew, Michael L. Klein, Anand Swaroop, 3 April 2023, eye science.
DOI: 10.1016/j.isci.2023.106417
This study was funded by the National Eye Institute, as well as Research to Prevent Blindness, the Retina Research Foundation, and the Macular Degeneration Center at the Casey Eye Institute.